|
The performance of a confocal microscope may be compromised due to the presence aberrations in the illumnation beam or some times due to the aberrations introduced by the target being imaged. By incorporating a programmable optical element in the laser beam path of a confocal microscope it is possible to have an illumination beam with a reconfigurable wavefront. Thus knowing the aberrations being introduced, it is possible with the programmable element to get a detection path that is free from aberrations.
Figure 2 shows the focus spots in the sample plane of a confocal microscope with a helical wavefront illumination beam. Due to the abberations present in the laser beam the focus spot (left) is not a perfect doughnut, while the focus spot (right)
is closer to a doughnut as the illumination beam has been partially corrected for aberrations using the programmable element.
In addition to correcting for aberrations the programmable diffractive element in the illumination beam path of the confocal microscope can be used to modulate the polarization profile. Thus one can do confocal imaging using various vector beams instead of the conventional scalar beam. We have developed a confocal system where the illumination beam can be periodically or aperiodically switched between different polarization states of the illumination beam. In Figure 3 we show confocal images of liquid crystal pixels in the ON or OFF state which correspond to orthogonal molecular orientaitons when the illumination beam is switched between X and Y polarizations after every line scanned. It is noticed that the same molecule with different orientations responds to X and Y polarizations in a different manner. This demonstrates the dependence of the spotanenous emission probability with the angle between the excitation field and the electric dipole associated with the moeculecule. More details about a confocal microscope with vecctor beam illumoination is available here.
|

Figure 2: The illumination beam spots with a helical wavefront in the presence of aberration (left) and after partial correction of aberrations (right).
|
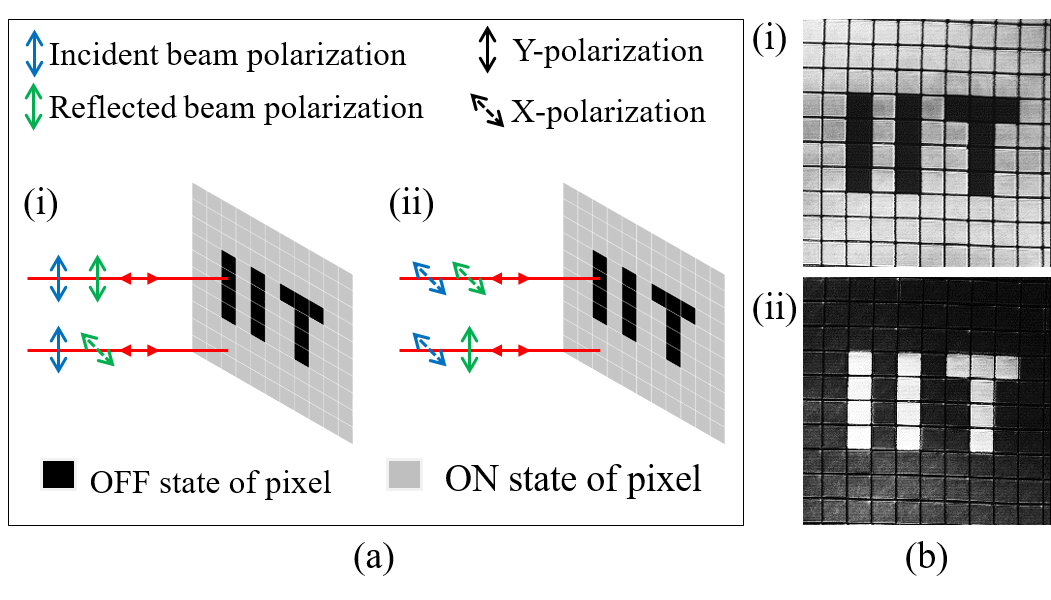
Figure 3: Schematic showing the change in polarization of the illumination beam by the ON/OFF pixels (left) and confocal images of liquid crystal pixels arranged as the "IIT" (right).
|
|